DDB Faculty in the News: Researchers reveal a new approach for treating degenerative diseases
Credit to: Harrison Tasoff
This is a story about proteins, how they malfunction, and what cells do to prevent that.
Proteins are the workhorses of life. Organisms use them as building blocks, receptors, processors, couriers and catalysts. A protein’s structure is critical to its function. Malformed proteins not only fail to carry out their tasks, they can accumulate and eventually gum up the inner workings of cells. As a result, misfolded proteins cause a variety of degenerative diseases, from Alzheimer’s and Parkinson’s to the blinding disease retinitis pigmentosa. These disorders are currently incurable.
A paper out of UC Santa Barbara reveals a new connection between a particular ion transport protein and the cell’s garbage disposal, which grinds up misfolded proteins to stave off their toxic accumulation. The results, published in Developmental Cell, identify a target for treating these debilitating conditions.
“By studying basic cell biology in fruit fly ovaries, we stumbled upon a way to prevent neurodegeneration, and we think this has potential applications in the treatment of some human diseases,” said senior author Denise Montell, Duggan Professor and Distinguished Professor in the Department of Molecular, Cellular, and Developmental Biology.
For 35 years, Montell’s lab has studied the movement of cells in fruit fly ovaries. It might seem esoteric, she is the first to admit, but it provides a fantastic model for cell mobility. “And cell movement underlies embryonic development, drives wound healing and contributes to tumor metastasis,” she explained. “So it’s a really fundamental cell behavior that we care to understand deeply.”
The setting and characters
The star of this paper is a gene called ZIP7, which encodes a protein of the same name. In previous work, Montell’s team came across a mutation in this gene that impaired cell mobility, piquing their interest.
The ZIP7 protein ferries zinc ions within a cell. These ions are exceedingly rare within the cytoplasm but abundant in proteins where they often form part of the architecture and catalyze chemical reactions. “ZIP7 is conserved in evolution from plants to yeast to flies to humans,” Montell said. “So it’s doing something really fundamental, because it’s been around for a really long time.”
ZIP7 is also the only zinc transporter found in the endoplasmic reticulum, a membranous structure where a cell makes proteins destined for the outer membrane of the cell or for secretion out of the cell. About a third of our proteins are made here.
If ZIP7 is our protagonist, then misfolded proteins and their disposal are the theme of the study. For proteins, function follows form. It’s not enough to have the right ingredients, a protein must fold correctly to function properly. Misfolded proteins are responsible for a host of diseases and disorders.
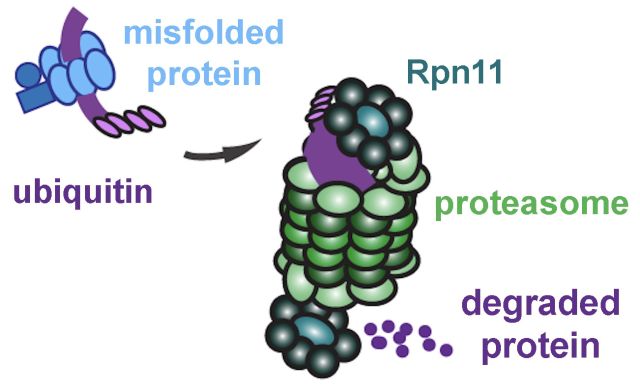
But proteins will sometimes misfold even in a healthy cell. Fortunately, cells have a quality control system to deal with this eventuality. If the error is small, the cell can try folding it again. Otherwise, it will tag the misfolded molecule with a small protein called ubiquitin and send it out of the endoplasmic reticulum (ER) for recycling.
Waiting in the cytoplasm are structures called proteasomes, the “garbage disposals” of the cell. “It literally chews up the protein into little pieces that can then be recycled,” Montell said.
“But if the garbage disposal gets overwhelmed — somebody puts too many potato peels in there — then the cell experiences ER stress.” This triggers a response that slows down protein synthesis (pauses our potato prep) and produces more proteasomes so that the system can clear the backlog of waste. If all this fails, the cell undergoes programmed death.
The plot thickens
Co-lead author Xiaoran Guo, Montell’s former Ph.D. student, saw that loss of ZIP7 caused ER stress in the fruit fly’s ovary. So she set out to determine if this stress was the reason the cells lost their mobility. Indeed, inducing ER stress with a different misfolded protein also impaired cell migration.
When Guo over-expressed ZIP7 in these cells, the backlog of misfolded proteins disappeared, the ER stress vanished, and the cells regained their mobility. “I was so surprised that I had to question myself if I had done everything correctly,” Guo said. “If this was real, just ZIP7 alone must be very potent in resolving ER stress.”
What’s more, the misfolded protein she used, called rhodopsin, contains no zinc in its structure. This led Guo to suspect that ZIP7 must be involved somewhere in the degradation pathway. Co-lead author, and fellow doctoral student, Morgan Mutch used a drug to block the proteasome from degrading misfolded rhodopsin and observed that this negated the beneficial effect of ZIP7. She concluded that ZIP7 must be acting somewhere before the proteasome munches up the misfolded protein.
The authors created four modified ZIP7 genes: two mutations disrupted the protein’s ability to carry zinc, while the other two left this unchanged. They discovered that zinc transport was critical in reducing ER stress.
At this point, a new character enters our story: the enzyme Rpn11, which forms part of the proteasome. Much like trying to stuff a large head of broccoli down the disposal, misfolded proteins with ubiquitin tags don’t fit into the proteasome. Rpn11 snips off these tags, enabling the misfolded protein to slip into the proteasome core for disassembly. Zinc is essential forRpn11 to catalyze the removal of ubiquitin.
“I was very surprised, and then excited, when I saw that increasing ZIP7 expression almost completely prevented the buildup of those ubiquitin-tagged proteins,” Mutch said. “We were expecting the opposite result.”
Mutch determined that ZIP7 was critical in supplying zinc to Rpn11, enabling it to trim the tags that label defective proteins so that they fit into the structure that actually breaks them down. Blocking the Rpn11 enzyme confirmed this hypothesis.
“That feeling when you discover something new, something no one has figured out before, is the best feeling for a scientist,” Mutch added.
A potential therapy
The results suggest that overexpressing ZIP7 could form the basis for treating a variety of diseases. For instance, misfolded rhodopsin causes retinitis pigmentosa, a congenital blinding disease that is currently untreatable. Scientists already have a strain of fruit flies with the mutation that causes a similar disease, so the team overexpressed the ZIP7 gene in these flies to see what would happen.
“We found that it prevents retinal degeneration and blindness,” Montell said. Every single one of the flies with mutant rhodopsin usually develops retinitis pigmentosa, but a full 65% of those with overactive ZIP7 formed eyes that respond normally to light.
Montell’s lab is now collaborating with Professor Dennis Clegg, also at UC Santa Barbara, to further investigate the effect of ZIP7 in human retinal organoids, tissue cultures that bear a mutation that causes retinitis pigmentosa. This project was originally funded by the National Institute for General Medical Sciences. For the next three years it will be supported by a $900,000 grant from the Foundation Fighting Blindness so Montell, Clegg and their colleagues can test the hypothesis that ZIP7 gene therapy will prevent blindness in retinitis pigmentosa patients.
What’s more, proteasome capacity declines as we get older, contributing to many classic signs of aging and increasing the probability of age-related degenerative diseases. Therapies targeting ZIP7 could potentially slow the development or progression of these ailments, as well. They’ve already yielded promising results extending fruit fly lifespan.
“This is a poster child for fundamental, curiosity-driven research,” Montell said. “You’re just studying something because it’s cool, and you follow the data and end up discovering something you never set out to study, possibly even a cure for multiple diseases.”